- When will AI surpass Facebook and Twitter as the major sources of fake news? – May 23, 2023
- The evolution of aging – May 7, 2023
- The (new) telomere theory of aging – April 15, 2023
In this philosophical analysis, I will debunk the top five myths about telomeres. These are as follows:
- Telomeres are protective caps at the end of chromosomes.
- Cellular replication causes telomere attrition.
- Telomere shortening cannot be the cause of aging, as some short-lived animals, such as mice, have long telomeres.
- The Hayflick limit evolved as a cancer suppressor.
- Telomeres do not encode.
Science and precise language
Before I debunk the top five telomere myths, allow me to explain why precision in the use of language is essential in science.
As a philosopher of science, the impreciseness of the language of cell biologists compared to that of other disciplines of the so-called hard sciences, such as physics or chemistry, came as a surprise to me. Many core biological concepts are not properly defined, and it is up to the individual scientist how to use particular terms in published papers. Take “chromosome,” perhaps the most important concept in microbiology, as an example. This is what we find on Wikipedia:
Some use the term chromosome in a wider sense, to refer to the individualized portions of chromatin in cells, either visible or not under light microscopy. Others use the concept in a narrower sense, to refer to the individualized portions of chromatin during cell division, visible under light microscopy due to high condensation.
I dare say, you’ll hardly find a central definition where some use a term this way and others use it another way in other hard sciences. Do you remember the heated debate about whether Pluto is a planet or not? Obviously, astrophysicists didn’t disagree about the empirical facts. The debate was only about the usage of the word “planet.” Imagine some use “planet” in a way that gives Pluto the full status of a planet, whereas others use the term dwarf planet instead. Most microbiologists probably wouldn’t care. Why not allow astrophysicists to use the term “planet” however they like? It is really just a matter of preference, right?
The problem is not just that sloppy definitions make communication difficult and promote misunderstandings. As we will see in the case of telomeres, inaccurate wording put the wrong ideas into the head of scientists, which often leads to the wrong theories.
Myth #1: Telomeres and protective caps
Capping chromosomes
Many scientific publications about telomeres begin with a definition similar to this one:
Telomeres are protective caps at the ends of chromosomes.
There are two problems with this definition. Telomeres are not protective, and there are no caps at the ends of chromosomes. I know this sounds hard to believe because you probably have seen an exact copy of this sentence countless times, but read on.
I already mentioned above that it is not even entirely clear what a chromosome is, but this is not my point here. The term “telomere” was introduced before it was discovered that chromosomes consist of DNA, many proteins, and other molecules that are attached to the double helix. “Telomere” literally means “end part,” which is why Hermann Muller termed the end of the chromosome this way in the 1930s.
Muller exposed chromosomes to X-rays and believed to have observed that the chromosome ends showed fewer alterations than the rest of the genome. He concluded that the telomere is a gene that seals off chromosomes, which protects them from X-rays. The myth of the telomere as the protective cap of the chromosome was born.
It is important to understand that scientists had no idea about the chemical structure of chromosomes and genes at this time. A gene was just anything that could be passed on to the next generation. From what we know today about chromosome ends, it remains a mystery how telomeres could have any protective power against irradiation. Nevertheless, Muller received the Nobel Prize for his work on the effects of radiation on mutagenesis, and you wouldn’t want to question a Nobel laureate.
The myth about the telomere as a protective cap started with a wrong theory, and it didn’t get much better for the next 90 years or so. Scientists tried to save Muller’s claim about the protective cap, even when evidence was mounting that chromosomes have no caps at all. Only much later did researchers discover that the end of the DNA molecule consists of a long repetitive nucleotide sequence (TTAGGG in vertebrates).
Microbiologists often use the term chromosome when they actually mean a DNA molecule. However, this sloppy use of scientific language is not my concern here, even though, psychologically, it might explain why the cap idea stayed alive. As it turned it out, the repetitive nucleotide sequence has helpers, the so-called shelterin complex.
The shelterin complex is actually the real actor here. These proteins bend telomeres into a so-called t-loop, which ensures that DNA damage repair proteins, which recognize loose ends of DNA, don’t mistakenly join the ends of sister chromatids or two chromosomes. Without the shelterin complex, telomeres are exposed like any other nucleotide sequence. However, a telomere without shelterin proteins and a t-loop is still a telomere because it still sits at the end of the DNA molecule. Some scientists call such telomeres “dysfunctional,” but this is a big misunderstanding because telomeres without a loop exactly fulfill the biological function that evolution had in mind. I’ll expand on this in more detail later in the post.
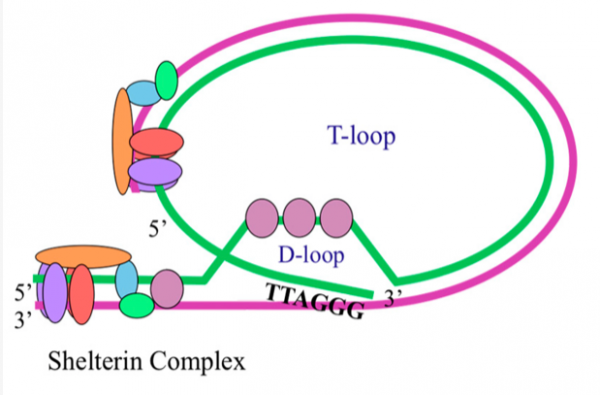
There are no caps at the ends of chromosomes. instead, the DNA molecule forms a loop with the help of the shelterin complex. This a modified illustration taken from here.
Of course, researchers know all this. However, because they were so used to referring to telomeres as protective caps at the ends of chromosomes, some scientists began to include the shelterin complex in their definition. Wikipedia uses the term “associated,” which elegantly avoids the question of whether the shelterin complex is a part of telomeres or not:
A telomere from Ancient Greek (télos) “end” and (méros) “part” is a region of repetitive nucleotide sequences associated with specialized proteins at the ends of linear chromosomes.
The reference to “associated” proteins with a sequence of nuclear DNA is indeed strange. I mean, there are countless proteins “associated” with every piece of nuclear DNA. Do we need a special term for the DNA that is wrapped around its “associated” histone? However, if we include the shelterin complex in the definition, this leads to inconsistent language. For instance, this is how Wikipedia defines “shelterin”:
Shelterin (also called telosome) is a protein complex known to protect telomeres in many eukaryotes from DNA repair mechanisms, as well as to regulate telomerase activity.
So, what now? The shelterin proteins protect the telomeres of which they are a part of? Thus, the shelterin complex protects itself? Are the telomeres the protectors, or do they need protection? Or perhaps both? Take your pick!
Well, in my view, all this talk about protection makes no sense because it assumes that the protection of chromosomes is the biological function of telomeres, which is actually not true. Again, I don’t plan on refuting any data or theory about telomeres. But let’s discuss first what scientists do not mean when they claim that telomeres are protective caps.
Protecting chromosomes from unravelling
The discussion about the language surrounding telomeres is a good example of how inaccurate language creates confusion in science. The metaphor of the protective caps has led scientists to compare telomeres with the aglets of shoelaces. This is how Nobel laureate Elizabeth Blackburn describes the metaphor in her book the Telomere Effect (p. 11):
Telomeres, which can be measured in units of DNA known as base pairs, are like the aglets; they form little caps at the ends of the chromosomes and keep the genetic material from unraveling.
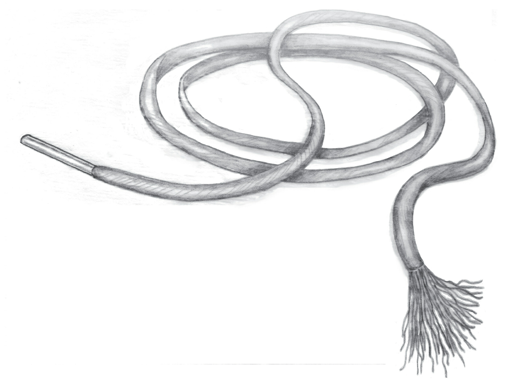
Nobel laureate elizabeth blackburn illustrates the protective role of telomeres in her book with the shoelace metaphor (p.15).
The problem with the shoelace metaphor is that many science writers take it literally and actually seem to believe that DNA starts to unravel after telomeres have shortened. Take this article as a “bad” example. The author really seems to believe that chromosomes unravel.
Many science writers take the shoelace metaphor literal.
Blackburn makes you believe that DNA unravels or unwinds with time, just as shoelaces would if the aglets were broken. In the case of chromosomes, “unravel” could mean two things: the unwinding of the double helix or the unravelling of the higher order structure of chromosomes, the so-called chromatin.
That the loss of telomeres could result in an unwinding of the two DNA strands is obviously nonsense. The long linear double helix in eukaryotic cells is an extremely stable molecule because of the sheer number of hydrogen bonds that hold the strands tightly together. And if, for some reason, a few base pairs at the end were separated for the fraction of a second, they would snap back to their original position in less than the blink of an eye. You need specific enzymes, so-called DNA helicases, to unwind DNA or high temperatures to denature the entire DNA molecule and not telomere removal if you want to “unravel DNA.”
The second idea, the unravelling of chromatin, makes no sense either. In shoelaces, no force exists between the different threads, which is why you need caps at the ends to prevent the threads from unravelling. But the loose ends of a DNA molecule have no effect on the hydrogen bonds and salt bridges between DNA and the proteins that create the higher order chromatin structure.
Thus, nothing that remotely resembles unwinding or unravelling occurs when telomeres can no longer form a t-loop. To understand what really happens to chromosomes when the t-loop is lost, we have to distinguish between normal healthy cells and unhealthy cells, that is, cells that do not behave the way they were programmed.
If everything goes as planned, the cell will commit suicide (more about this later) when the t-loop can no longer form. Case closed. No chromosome lost its protection, no chromosome unraveled or became unstable. The chromosomes of the corresponding cell just cease to exist.
If the suicide path is blocked for some reason, the DNA repair machinery sometimes fuses sister chromatids or two different chromosomes, resulting in unstable chromosomes. “Unstable” just means that all kinds of weird and unpredictable things can happen, like fused chromosomes getting ripped apart at random positions during segregation in mitosis, only to be fused again with different chromosomes after the division is completed (breakage-fusion-bridge cycles). The cell continues to divide multiple times, and an increasing number of chromosomes stick together. In the end, you can get something like “chromosome spaghetti.”
Telomere loss can create chromosome spaghetti.
In scientific papers, you will never find the claim that telomeres protect chromosomes from unravelling. However, because scientists love the shoelace metaphor so much, you will find a myriad of incorrect descriptions on the web. Later in this article, it will become clear that it is also not the function of telomeres to protect chromosomes from instability. But let’s first discuss another way telomeres might protect chromosomes.
Protecting genes from deletion
Scientists who claim that telomeres protect chromosomes also often just mean that telomeres protect genes of the subtelomeric DNA from deletion when the double helix duplicates. As explained below, when DNA duplicates, a few nucleotides at the end are always removed.
However, cells stop dividing long before this can become a real issue for the subtelomeric DNA. If the t-loop is gone, the result is not that the rest of the DNA is no longer protected from deletion, simply because the cell will no longer duplicate. No DNA duplication, no end replication problem (see below), and subtelomeric genes are therefore not in danger of being deleted. Thus, in reality, not even in this weak sense do telomeres protect DNA.
However, my main argument against the claim that telomeres protect DNA from deletion during replication is that it is hard to believe that evolution came up with this insanely complex telomere maintenance system (I only scratch the surface in this article) just to ensure that no genes at the end of DNA are deleted during replication. Simply adding the lost nucleotides after duplication would be enough. Thus, the real biological function of telomeres must be something very different, something that has nothing to do with the protection of chromosomes.
To sum up, neither chromosomes nor DNA feature any kind of caps. A cap, like the aglet on a shoelace, would be of a different material that protects the DNA by entirely sealing it off from its environment, as Hermann Muller, the unfortunate inventor of the term, might have imagined it. Such chromosome caps do not exist in eukaryotes.
However, some prokaryotes with linear DNA actually do have caps made of proteins. If Mother Nature wanted only to protect the ends of chromosomes, this would be the way to go. Whenever a piece of DNA needs protection, the solution is always a cap made of proteins or other molecules that seal off this nucleotide sequence from its environment.
And just because telomeres are at the end of DNA does not mean that this molecule has caps. If the telomeres are lost, does the DNA then have new caps? I guess not. The end of something is not necessarily a cap. The DNA molecule is a chain of nucleotides that reaches from end to end without being terminated by caps.
The DNA terminus just forms a loop to avoid loose ends, which would attract DNA repair enzymes that fuse DNA ends. But of course, the shelterin complex is no cap either, as illustrated in the image above because the proteins don’t seal off the nucleotide sequence to which they are attached.
By the way, this solution is not unlike that found in most prokaryotes where DNA builds a ring for the same purpose. The loops in eukaryotes are just smaller and don’t reach from end to end.
Myth #2: Telomeres and attrition
This is my favorite telomere myth. If you are interested in theories about aging, you will have come across the term “telomere attrition” many times. Let me give you an example: This is from Blackburn’s book (p. XV):
Telomeres throughout the body shorten as we age, and this underlying mechanism contributes to most diseases of aging. There are other ways cells become dysfunctional or die early, and there are other factors that contribute to human aging. But telomere attrition is a clear and an early contributor to the aging process, and—more exciting—it is possible to slow or even reverse that attrition.
And this is how dictionary.com defines attrition:
a wearing down or away by friction
The keyword here is “wearing down.” In the literature, you will often find terms like “telomere wear down,” “telomere erosion,” “telomere deterioration,” or even “telomere fraying.” What I find fascinating here is that a scientist who received a Nobel Prize for her research about telomeres doesn’t bother to explain in her book about the very same topic what is actually happening here. I mean, this is the one and only thing that is really fascinating about telomeres, and this discovery would have been really worthy of a Nobel Prize.
I can’t go into the details in this blog post, but attrition is certainly not the reason telomeres shorten. The real cause is the so-called end replication problem. Interestingly, this was discovered by the theorist Alexey Olovnikov and not by an experimentalist. It is a general problem in microbiology that theorists are underrepresented. In the absence of a theoretical framework, experimentalists are often misguided about what they see under the microscope, which then results in inconsistent nomenclature and explanations.
The simplified description of the end replication problem is this: DNA polymerase, the enzyme that synthesizes new strands during genome replication, needs a primer attached to DNA as a starting point. Normally, this primer is removed and replaced with the correct nucleotide sequence after the copy process is completed. However, when the lagging strand duplicates, the last primer is not replaced, and the DNA to which the primer was attached to (and the DNA behind it) is essentially cut off. The result is that telomeres are cropped a little with every cell division.
The video below illustrates this better than words can. Note that new research indicates that the video is not entirely correct because the last primer does not sit at the end of the DNA. However, I couldn’t find a newer illustration, probably because scientists don’t really understand the details.
The important takeaway is that nothing even remotely resembles attrition when telomeres are shortened. “Attrition” implies that some kind of grinding is going on, with telomeres wearing down because a cellular process causes stochastic, collateral damage. Your shoe soles wear down because of attrition when you walk. Telomeres don’t wear down when cells multiply.
Of course, the use of “attrition” is inspired by the wear and tear theory of aging, which claims that metabolism causes gradual deterioration in cells and tissue. In short, according to this increasingly rejected theory, your body wears out over time simply because you use it. (So better not exercise because this causes lots of “wear and tear” and “attrition” on your sneakers, your cells, and your entire body. .;-) )
The opposite to attrition happens with telomeres during DNA replication: A precisely programmed procedure removes a distinct sequence of DNA in a very predictable manner. Obviously, cell division does not cause telomere attrition through wear and tear. Instead, a programmed process crops telomeric DNA, or to be more precise, cell division triggers a telomere countdown.
Again, this is just about words. Neither Blackburn (who, by the way, does not favor the wear and tear theory of aging) nor any other scientist would disagree that the end replication problem is the “problem” here.
It is important to note that some kind of “telomere attrition” actually exists. However, this kind of attrition is not caused by DNA duplication. Stochastic events that occur because of cell metabolism can damage telomeres. For instance, oxidative damage can cause an accumulation of single-strand breaks, which persist longer in telomeres because this kind of damage is poorly repaired in telomeric DNA. A telomere can even be entirely lost because of real “wear and tear” and “attrition,” which can cause sister chromatid fusion and cell death.
There is evidence that an additional function of telomeres is to detect stochastic damage caused by cellular stress (1, 2). This is real telomere attrition that needs to be distinguished from telomere countdown.
Telomere countdown is a genetically programmed process, which serves an essential biological function, whereas telomere erosion or attrition is caused by biologically harmful stochastic events.
As we will see below, the “end replication problem” is also a misnomer because telomere countdown is not a problem but a brilliant solution that evolution found for a real problem, that is, how to count the number of cell divisions. Why this is significant will become clear when we talk of mice and men.
Myth #3: Telomeres and mice
As mentioned above, inaccurate language often puts flawed ideas in the heads of scientists. One objection against the telomere theory of aging is that mice, which live only up to 3 years when in the relatively safe environment of a lab, have much longer telomeres than humans. In addition, somatic mouse cells express telomerase, an enzyme that can extend telomeres, which is usually not present in human somatic cells.
It seems scientists who use this argument never really understood the point of this theory. They appear to believe that the supporters of the telomere theory of aging claim that telomeres are some kind of life elixir and as this life-giving substance wears away by attrition, signs of aging begin to manifest. Of course, this is absurd!
Even scientists who favor the telomere theory of aging often misrepresent it by using the wrong language. A term that you find in almost every paper about telomeres is telomere dysfunction. Scientists like to explain that because of telomere attrition, telomeres eventually become too short or reach a critical short length, which results in telomere dysfunction, and so telomeres can no longer protect the chromosomes, which then become unstable and so the cell dies. This description uses erroneous language!
Imagine you set a timer because you want to boil your morning egg. When the alarm goes off, you exclaim “oh, my timer has become dysfunctional, my egg is no longer protected.” Of course, a ringing alarm is the best proof you can get that your timer is fully functional, and it is also clear that the timer never had the capability to protect your egg. The only power of the timer is to set you in motion and come to the rescue of your breakfast before it starts to disintegrate in the boiling water.
Thus, we can conclude egg timers don’t protect eggs, and likewise telomeres do not protect chromosomes. The biological function of the telomere countdown is to tell the cell when it is time to commit suicide and inform nearby macrophages that breakfast is ready.
The correct microbiological language when describing the function of telomeres is this: The function of the telomere countdown is to trigger a timer that goes off when a telomere has reached exactly the correct preprogrammed length, which then prevents shelterin proteins from forming a t-loop. The ends of the telomeres become exposed, which attracts DNA repair enzymes that fix double strand breaks. At this point, the telomeres have proved that they are fully functional, that is, they have fulfilled their evolved task. As the DNA damage repair machinery believes it has detected an intrachromosomal double-strand break that cannot be fixed, it triggers the so-called p53 response, which tells the cell to stop dividing (cell cycle arrest), which eventually leads to cell death (apoptosis).
In most cases, this postmitotic cell signals the need to be phagocytosed, that is, to be gobbled up by a macrophage. The cell has reached the end of its genetically preprogrammed lifespan and needs to be removed from its ecosystem, which you call your body. A dead cell is dangerous because its genetic material and proteins would confuse the immune system once the cell started to disintegrate and all its contents spilled into the intercellular space. Thus, the main function of telomeres is not to protect chromosomes from becoming unstable but to make sure that the cell with all its chromosomes is destroyed when its time has come.
Once you understand that the telomere countdown is essentially a timer with the sole function to operate a switch that tells the cell that it is time to make space for the next generation, it becomes clear that the absolute number of nucleotides in the telomeres of a certain species is as unimportant as the number of gear teeth in different types of clocks. All that matters is that all eukaryotic cells feature a mitotic clock that limits the number of cell replications. Even single-celled yeast divides only up to 25 times, and mouse cells stop replicating after 15–20 cycles (at least in vitro). As it turns out, the telomere countdown timer in mice ticks 100 times faster than in human cells.
The ability of human cells to replicate 40–60 times has been known since 1961 when Leonard Hayflick and Paul Moorhead debunked the myth spread by Nobel laureate Alexis Carrel that cells grow indefinitely. This myth was kept alive for half a century, even though Carrel’s experiment was never replicated.
The Hayflick limit, as we call it nowadays, puts the first nail in the coffin of neo-Darwinist theory, which claims that life constantly tries to optimize its biotic potential, that is, to maximize the replication rate of genetic information. Programmed cellular suicide, particular of unicellular eukaryotes, and aging in general do not fit well in the neo-Darwinist’s world.
The neo-Darwinist needed an explanation of this apparently altruistic behavior of cells. In the case of the Hayflick limit, a scapegoat was quickly found. Cancer, the scourge of humanity, was identified as guilty.
Myth #4: Telomeres and cancer
Cancerous cells mutate in a way that allows them to divide in an uncontrolled manner indefinitely, destroying tissue along the way. The fact that cancer cells are immortal is the alleged reason why normal somatic cells evolved to be mortal. (On a side note, I’ve always wondered how the supporters of the wear and tear theory of aging explain that immortal cancer cells don’t “wear out” over time.)
The likelihood that a normal cell acquires all the mutations required to become cancerous increases with time and with the number of cell divisions. Thus, evolution came up with the Hayflick limit to get rid of old somatic cells before they wreak havoc, according to the theory. The fact that cancer cells often express telomerase, the enzyme that extends telomeres, is one of the main facts put forward as evidence.
Normal human somatic cells usually don’t express telomerase. Only during embryogenesis, in germ cells, and to a much lower extent in stem cells, is this enzyme complex active under normal conditions. In cancer cells, telomerase expression indefinitely resets the telomere countdown timer, and thus the p53 death switch is never flipped. The Hayflick limit is outmaneuvered, making cancer cells immortal.
This all sounds very plausible, except that it is not. The main flaw in this thinking is that because cancer causes so much trouble for us humans, it also must be a big problem for benevolent Mother Nature. This is not the case for two reasons:
First, it is wrongly assumed that everything that is harmful for an organism is something that evolution wanted to avoid but failed to do so for some reason. I will cover this false premise in more detail in my next article. Once you can admit that Mother Nature actually wants you to die at a certain age because all that it is relevant for her is to accelerate evolution (evolution is all about innovation and therefore about replacing the old with the new), you see that any cause of death becomes useful at a certain point. All that Mother Nature needs to do is to take all possible causes of death into account, so that the aging rate for a particular species can be optimized. Predators, pathogens, shortage of resources (food, water, etc.), intraspecies competition, accidents, failed evolution experiments (unfortunate mutations), and last but not least cancer are all possible avenues to death.
Death because of aging is a way to fine-tune the optimal average lifespan of a species for its particular niche. Therefore, the cancer prevention machinery just needs to be adapted to enable the species to reach its optimal lifespan. Not more and not less, just the right dosage of cancer risk that fits the optimal lifespan of the species will do.
Second, Mother Nature has proven many times that cancer is not a big deal for her at the microbiological level. The fact that our scientists struggle so hard in their fight against cancer only demonstrates how primitive our bioscience still is. Evolution has solved this problem, probably when the first multicellular organisms emerged 1.5-2 billion years ago. I am talking of the fact that some species have no or only a very low cancer risk.
Take whales as an example. A whale has 1,000 times more cells than a human, yet its risk of cancer is lower than that in humans. Mice, on the other hand, have 1,000 times fewer cells than humans but are much more susceptible to cancer than we are.
More cells mean a higher probability that a cell acquires the random mutations needed to become cancerous. A single cancerous cell is enough to develop a tumor because it can replicate indefinitely. Thus, the bigger the animal, the higher the probability that one of its cells will become cancerous.
So, how is it possible that massive animals have a lower cancer risk? This is known as Peto’s paradox. Notice that it is not only the number of cells that is relevant but the lifespan of the animal. The fact that these giants live much longer than most smaller animals means that their cells have more time to mutate.
This is another hint that it is not the job of the telomere countdown and the Hayflick limit to prevent cancer. But there is more. Galápagos tortoises not only live a long time, they appear to very rarely get cancer. And now it comes–the cells of these long-lived reptiles divide up to a whopping 130 times. That’s roughly 10 times as often as in mice, the short-lived mammal with one of the highest cancer risks.

Galápagos tortoises age slower than humans, partly because of their generous hayflick limit.
If the Hayflick limit is supposed to lower the cancer risk, then how come that animals with a much higher statistical chance to develop cancerous cells and a much more generous Hayflick limit have a significantly lower cancer risk? Why do cells of Galápagos tortoises not wreak havoc in 100 years, even though their cells replicate 130 times? I call this the telomere-cancer paradox.
The good news is that the solution to these two apparent paradoxes is already known. It is not telomeres and the limit on cell cycles that prevents tumors in these animals but how their cells detect that they are at risk of becoming cancerous. A key role is played by p53, the protein that we met before.
Just in case you wonder where the name comes from, “p” stands for protein, and “53” is supposed to be its mass in kilodalton, which was actually incorrectly measured. So, we just encountered another misnomer, which doesn’t bother the microbiologist that much. The corresponding gene was named TP53 where “T” refers to tumor, which is yet another misnomer because the gene has other core functions, and tumor suppression is just one of its jobs.
The interesting thing about p53 is that specific modifying groups can be added to the protein in response to different signals, which alter its function in many ways. However, all its functions are tied in one way or another to cell cycle control, that is, the management of the different stages of cell division. The protein can pause the cell cycle, for instance, if DNA needs repair. If the repair fails, p53 can initiate cell cycle arrest and senescence. A side effect of this function is that mutations that could possibly create a cancerous cell can be detected, and p53 will then order the cell to commit suicide (apoptosis).
Thus, in some cases, TP53 can act as a tumor suppressor. The trouble begins if TP53 mutates, with the result that the damage control machinery is itself damaged. At first, this sounds really bad. How can you prevent a cell from becoming cancerous if the very mechanism that is supposed to detect mutations can simply be disabled by a random mutation? Notice that TP53 doesn’t even have to be modified in a specific way. All that is needed is that its function is disrupted, and as we know, destroying is much easier than building or repairing.
However, there is a simple solution to the problem. Mother Nature just did what every engineer would do in such a situation. If your storage media or information channel is unstable, all you have to do is create backups. If you are worried that your backup may be destroyed too, you create more backups of the backup. The number of backups depends on how paranoid the engineer is or, to formulate it more positively, how much certainty the task requires.
In the case of the African forest elephant, another gigantic animal with a low cancer risk, Mother Nature was very paranoid and donated up to 24 copies of TP53. As Asian elephants are a bit smaller, 9-20 backups are good enough. And now guess how much Mother Nature is worried about you? The crowning of creation is worth only two copies.
I have to add here that TP53 is mutated in only 50–60% of all cancers, which makes the situation worse because a tumor can develop even if TP53 is just fine. On the other hand, Mother Nature obviously has a few more tricks up her sleeve to deal with cancer. For instance, the long-lived blind mole rat doesn’t have extra copies of TP53 and still is extremely cancer resistant.
What all this tells us is that the Hayflick limit would be a very dumb way to prevent tumors, and it is not just that it is extremely trivial to outsmart this alleged tumor suppressor by expressing telomerase.
Imagine, on your 50th birthday, your government sends you a gift. You get a gun with a little letter where your president explains that new research has proven that people older than 50 are much more susceptible to becoming psychopaths and mass murderers. You’ve probably already done your duty and produced offspring, so putting a bullet in your head right after your birthday cake would be a wonderful altruistic service to your great country. It’s better than becoming a mass murderer, even if the chance appears to be very low to you, right?
Mother Nature is very cruel, that’s for sure, but she certainly is not stupid. Killing a perfectly healthy cell just because one of 30,000,000,000,000 cells in your body might become rogue is not really efficient. Mother Nature never kills without a real, concrete purpose. The turnover rate in a human body is 4 million cells per second! The vast majority of cells are eliminated for a particular, vital reason. For instance, red blood cells don’t feature a nucleus and therefore cannot repair themselves, and T-cells of the adaptive immune system have to be eliminated if they recognize self-antigen to avoid autoimmune disease.
In general, somatic cells of long-lived animals with a low cancer risk are much more sensitive to any kind of unrepairable damage, and only those cells are then terminated, which obviously is a much more efficient way to deal with cancer than proactively killing every cell that reached a certain age.
That the Hayflick limit has nothing to do with tumor prevention was clear long before we learned that there are much more efficient ways to restrain unruly cells. The fact that unicellular eukaryotic organisms also only divide a limited number of times indicates that this restriction serves a very different purpose. Cancer in the context of a single-celled organism does not make any sense. I mean, the neo-Darwinist keeps telling us that evolution is all about individual fitness and replicating selfish genes, and what could be more fit than a cell that creates copies of itself like wild without any limits or restraints?
We’ll get a glimpse of why the Hayflick limit evolved when we discuss that, contrary to the established manner of speaking, telomeres have important encoding to do.
Myth #5: Telomeres and encoding
In older textbooks, you can still can read that telomeres are not transcribed. This claim was falsified when scientists discovered a couple of years ago that telomeric DNA is used to build an RNA molecule termed TERRA. TERRA has critical functions in telomere maintenance, which I won’t discuss here.
Cell biologists wouldn’t call this “encoding” because the central dogma of molecular biology reserves this term for genetic information that makes it all the way from DNA to protein, that is, a gene that “encodes” must first be transcribed and then translated. RNA that is not translated to build a protein is called non-coding RNA.
However, considering that we now know that non-coding RNA performs essential biological functions, this distinction does not make much sense anymore. The encoding of proteins is just a bit more complicated than in the case of RNA. Nevertheless, this is not my point when I claim that telomeres actually do encode.
What a cell does, that is how it behaves, what proteins and RNA it builds, and how it controls and manages all the other molecules with biological functions depends on the signals it receives from its environment. In a multicellular organism, most of these signals come from other cells where neighboring cells play crucial roles in determining the cell’s identity (e.g., a skin cell or liver cell).
You could say that the location in the organism determines cell identity. However, this is only half of the story. Organisms change with time, and we call this aging. Because everything that happens in an organism is determined by the behavior of its cells, the way cells change their behavior over time is crucial. Thus, not only “space” determines the identity of a cell, time is just as important. Space and time determine cell identity.
If you want to know what time it is, you need a clock, and this is the main function of telomeres. Hence, telomeres encode time. Of course, time is not measured in minutes or hours but in the number of cell cycles, the unit of time measurement in cell biology. Once you know the average length of the telomeres of a particular species, you can decode the state of the countdown timer if you measure the telomere length. You can then use this information to calculate how many times a particular cell has replicated. Therefore, it is not the nucleotide sequence of a telomere, but its relative length, that encodes time, the age of the cell.
However, when it comes to the age of tissue or the entire organism–we call this the biological age in contrast to the organism’s chronological age, which we measure in years–the telomere length or even the average length of all cells cannot be the clock the organism uses to measure its age. It’s a bit more complicated. Opponents of the telomere theory of aging often attack this hopelessly oversimplified version and conduct naive experiments where telomere extension does not result in significant age reversal of the organism.
In my next post, I will outline in more detail how the telomere countdown timer changes cell identity over time and how this causes aging at the system level. Only the telomere theory of aging can explain all hallmarks of aging. Subscribe to the newsletter if you want to be informed when I publish the article.