- When will AI surpass Facebook and Twitter as the major sources of fake news? – May 23, 2023
- The evolution of aging – May 7, 2023
- The (new) telomere theory of aging – April 15, 2023
The hallmarks of aging
Sinclair claims no less than that his Information Theory of Aging explains all hallmarks of aging. Let me give you his complete list (p. 17):
- Genomic instability caused by DNA damage
- Attrition of the protective chromosomal endcaps, the telomeres
- Alterations to the epigenome that controls which genes are turned on and off
- Loss of healthy protein maintenance, known as proteostasis
- Deregulated nutrient sensing caused by metabolic changes
- Mitochondrial dysfunction
- Accumulation of senescent zombielike cells that inflame healthy cells
- Exhaustion of stem cells
- Altered intercellular communication and the production of inflammatory molecules
Let’s have a look at Sinclair’s unified theory of aging.
The Information Theory of Aging
The short version of the Information Theory of Aging is this:
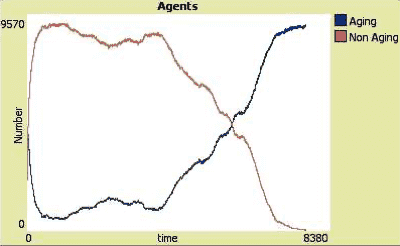
Youth → broken DNA → genome instability → disruption of DNA packaging and gene regulation (the epigenome) → loss of cell identity → cellular senescence → disease → death (p.41).
Others have proposed that DNA damage causes aging. However, this is not Sinclair’s point. First, we are dealing here with a particular form of DNA damage, that is, DNA double-strand breaks. These breaks happen trillions of times every day in an organism the size of a human. Most of them occur (accidentally) when cells multiply, from natural radiation, or simply from chemical reactions inside the cell.
Thus, broken DNA is a very common phenomenon, and you shouldn’t worry too much about the countless DNA breaks happening right now inside your body while you read this blog post. Our cells are well prepared for this kind of damage and repair these breaks as fast as they occur.
However, as you can imagine from this sheer number of breaks occurring during the lifetime of an organism, sometimes things go wrong during the repair process. This is the crucial point of Sinclair’s theory.
The best way to understand his theory is to look at a concrete example. The Sir2 protein in yeast cells is one enzyme responsible for repairing such DNA breaks.
However, this protein has a second job. It silences mating-type genes by removing acetyls from histones. If Sir2 didn’t remove these acetyls, the DNA packaging at this location would loosen, and the corresponding genes would turn on.
A crucial point here is that during the time Sir2 is busy repairing a DNA break, both female and male genes turn on, the cell loses its identity and becomes infertile. As odd as this may sound, the coupling of these two functions of Sir2 has an important biological reason. Yeast cells need to avoid mating until the DNA break is fixed because reproduction would most likely fail otherwise.
Now comes the important part that, according to Sinclair, causes aging. Sometimes Sir2 doesn’t find its way back to its original position, and the cell becomes permanently sterile and therefore senescent. If you check the list of the aging hallmarks above, you will find it includes cell senescence.
Sir2 (the corresponding gene is SIR2) belongs to a class of proteins, the so-called sirtuins, which are Sinclair’s major research focus. In mammals, seven such genes exist (SIRT1–SIRT7), and their main task is cellular regulation.
Regulating gene expression is the job of the epigenome. Thus, the core of the Information Theory of Aging is that epigenetic noise (like when Sir2 doesn’t find its way back from its repair job) results in a loss of epigenetic information (the cell no longer knows if it is male or female in the example here).
Of course, the other sirtuins have other epigenetic functions, and epigenetic noise can mean different kinds of epigenetic damage. However, the main point here is that aging occurs when genes that are supposed to be silent (or active) in a certain cell type are turned on (or off). In other words, the pattern of gene expression becomes abnormal, the cell malfunctions and loses its identity. As the number of these malfunctioning cells increases during a lifetime, the entire organism “malfunctions” more and more, which we perceive as aging. Once the number of abnormal cells surpasses a certain threshold, the entire organism fails, and we call this death.
Because of their important role in aging, Sinclair calls the sirtuins “longevity genes.” There are other longevity genes, such as mTOR and AMPK. It is common to all longevity genes that stressors (DNA breaks, inflammation, food shortage, etc.) activate them. This forces the system to hunker down (for instance, to stop mating) during the stressful period and focus on dealing with the stressor. Thus, the reason why stressors such as calorie restriction increase longevity is because cells take more care of damage in those times.
Telomeres and the Information Theory of Aging
Sinclair doesn’t outline how his theory explains all the hallmarks of aging mentioned above (although some explanations are self-evident). However, he briefly discusses one popular approach: the telomere theory of aging.
You’ve probably heard that the telomeres located at the end of the chromosomes shorten with every cell division (mitosis). Once the telomeres have shortened to a certain length, they lose their histone packaging. The end of the DNA becomes exposed, and the cell interprets this as a DNA break.
The problem is that there is no second piece of DNA to reattach because we are at the end of the DNA molecule. The result is that epigenetic factors such as the sirtuins leave their main post forever because a dangling piece of DNA they can’t rejoin fools them. The cell shuts down and permanently stops dividing, which nicely explains the Hayflick limit.
Thus, Sinclair’s theory doesn’t contradict the telomere theory of aging. It rather explains why telomeres play an important role in aging. However, the Information Theory of Aging also claims telomere shortening is just one factor or hallmark of aging.
Conclusion and questions
Sinclair clearly is in the camp of those scientists who support the damage theory of aging. His theory differs from other similar approaches only in the emphasis on epigenetic damage. If I understand his main point correctly, epigenetic noise or information loss is the major cause of aging. My conclusion is that once a cell is no longer able to express the right set of genes, other forms of damage accumulate in the cell simply because the repair mechanism is impaired.
On first sight, this all seems very plausible. However, I still have a hard time believing this is the entire story. I have two major problems with Sinclair’s theory and any theory that claims the major cause of aging is just accumulated damage.
First, why don’t cells simply make more repair enzymes? For instances, if Sir2 doesn’t sometimes find its way back to its original position, why not simply make more Sir2? In fact, Sinclair has shown that adding an extra copy of SIR2 increases the lifespan (the number of divisions) in yeast cells (p. 48).
Sinclair’s resolution of this contradiction is that the advantage of living longer does not justify the additional energy costs of making more Sir2. This might sound plausible. But did anyone really do the math here? How much energy does it actually cost to make a little more Sir2, and what exactly would be the biological advantage if a yeast cell can create more copies of its DNA because it lives longer?
The thing is that cellular damage is the major obstacle life faces. As mentioned above, DNA breaks alone occur trillions of times every day in our bodies. If cells go through so much effort to repair all of this damage, why not take this tiny extra step and also repair the damage that causes aging?
The fraction of unrepaired damage must be incredibly small compared to the damage cells actually repair every second in our bodies. Wouldn’t the tiny extra energy needed for this justify the enormous advantage to staying strong and healthy—at least until the organism dies of other natural causes (predators, infections, accidents, etc.)?
My second problem with the damage theory of aging is that it has a hard time explaining why organisms exist that don’t age at all or can even reverse aging. Why exactly do these creatures invest this extra energy to avoid aging whereas most other organisms do not? If evolution already figured out how to get rid of all cellular damage, why hasn’t this wonderful technology found its way into all organisms?
Because of these unanswered questions, I still feel that aging is not a bug but an important feature of biological evolution. My guess about the main biological function of aging is that it increases diversity in the gene pool. Genetic diversity is perhaps the most important factor when it comes to combating pathogens. So why waste the limited available resources to add the same or similar genetic variations again and again to the gene pool?
In other words, evolution not only favors species that maximize the quantity of their offspring, but quality is just as important. Whereas aging reduces the quantity of offspring, it enhances the quality of the gene pool. Thus, I think aging improves evolvability.
Of course, I am only an interested layman and no match at all for a high-class scientist like David Sinclair. Thus, my criticism should only be seen as the questions of a puzzled student. If you can answer my questions, feel free to post a comment below.
In my next post, I will discuss the second major topic of Sinclair’s book—the question “why we don’t have to age.”










Said another way short lived animals can evolve more quickly than long lived animals. However humans could not have evolved as we have with a lifespan of a mouse. On the other hand long lived species are at greater risk of extinction due to a rapidly altered environment as befell the dinosaurs. Nevertheless biology is infinitely mailable so aging is solvable. However if challenges prove to be to great in pure biology, which I think is unlikely, evolving to cyborgs or purely nonbiological solutions will be the alternative. One way or another the future is exciting.
Chris, exactly! A tradeoff exists between the average lifespan of a species and evolvability. Short-lived animals evolve faster, but can have less offspring. However, I think things are much more complex as many factors play a role here. For instance, the optimal lifespan depends on the niche, the available resources, the level of intra-species competition, the number of offspring, the number of predators and many more things.
This is why I am always confused if scientists who support the damage theory of aging oversimplify things when they claim that evolution didn’t need to find a solution for aging because animals die anyway of other causes.
At best, this is a nice philosophical idea but no scientific theory. A real theory needs to take all factors into account and then really do the math. And my guess is that it will then turn out that aging is programmed and not just the result of accumulating damage. That is, aging is a programmed, biological developmental process that has evolved to optimize the lifespan of a species.
Dear Michael,
Speaking of contradictions, as you well put:
“Of course, I am only an interested layman and no match at all for a high-class scientist like David Sinclair.”
But then you say:
“…scientists who support the damage theory of aging oversimplify things…” or “At best, this is a nice philosophical idea but no scientific theory.”
Now I find extremely presumptuous that you, who have a superficial understanding on natural sciences compared to a Harvard professor like David Sinclair, would call his lifetime work “a nice philosophical idea but no scientific theory”. This lack of humbleness and respect is where you loose all capacity to argue on his theory.
First of all, Lifespan the book, is not a scientific paper. If it was, it would probably be the size of an encyclopedia. It’s a very superficial way of outlining his theory for laymen to understand.
Then you say “the optimal lifespan depends on the niche, the available resources” – this is oversimplifying things. Who told you that? Common sense? Everyday knowledge? A youtube video? Of course there is way, way more to why a species has the longevity it has, beyond available resources. Darwinism is falling off the shelf as we know it. It’s been proven wrong again and again and it’s exactly one of the points of David’s book (which I bought the day it came out) when he says that as scientists try to adjust the model as little as possible while trying to address unanswered questions, the model enters in crisis mode and that’s usually when old scientists try to resist changes proposed by new scientists… This idea that we are a mathematical function of the environment is outdated and primitive. In fact one of the problems of old theories is being too attached to Newtonianism and materialism and to “doing the math” as you state. Doing the math is no longer enough when you realize you live in a reality with objective and subjective components. Do you think you will ever explain subjective experience mathematically? Good luck with explaining the numbers of preferring yellow to purple. 😉
No, where science is moving to is to models based on information but where free will and subjective interpretation of objective information, render subjective experience possible. As Immanuel Kant stated, certain aspects of reality (the categorical imperatives) are a priori structures of the mind, and not objective aspects of reality. Reality as we know it is a byproduct of the mind and its structures so, the mind resides outside reality. Reality is rendered, therefore created by the mind/consciousness… It’s not consciousness that is created by reality. Even mathematically you know you cannot contain the supra-set inside the sub-set. It’s pure logic. Consciousness is fundamental… Not matter. So be careful with trying to explain everything mathematically, materialistically and in an excessively deterministic way. Use logic… Not Darwinism. Aging is way more than a consequences of niche and resources.
Miguel, thank you for your comment.
First of all, as someone who has been trained in the philosophy of science, the history of science and quite a few different natural sciences, I can tell you that all reputable scientists make many wrong claims throughout their careers. Thus, I don’t think it is a good idea to simply believe everything just because it comes from a Harvard professor. It is a core value of science to question scientific theories again and again and no good teacher would want his students to buy just everything he or she says only because of his reputation.
In particular, if you look at the science of aging you will notice that many high profile scientists contradict each other about very basic things. Thus, it is safe to say that many, if not most of the claims currently made in this field are oversimplifications or simply wrong.
Secondly, you really don’t need to be a gerontologist to see that the damage theory of aging is not a scientific theory. By saying that is a philosophical idea I didn’t mean to belittle the theory. Philosophical ideas can often guide scientists to create good scientific theories. However, in some case they also lead to wrong theories.
The reason why the damage theory of aging cannot be a scientific theory is because “damage” is not an objective term. If something is damaged or not is subjective. Natural sciences can only deal with objective terms because theories have to be tested empirically.
Let me give you an example. Getting grey hair is a sign of aging. You can argue the reason why you get grey hair is because pigments cells die when you age and thus the process of colouring your hair has been “damaged.” You could also say the pigment cells die because they have been “damaged.” However, grey hair also has an important sociobiological function. It marks members of a community as old and wise and can therefore help the community to master difficult situations if younger members get advice from those members with grey hair. Hence, getting grey hair increases the likelihood that your offspring survives.
Thus, the question if the pigment cells are damaged or just have undergone a normal developmental process depends on your subjective point of view. From the point of view of evolution, everything works perfectly fine and as planned. From your point view (if you don’t like to get grey hair), your pigment cells have been damaged.
In fact, there is no way to empirically falsify the damage theory of aging. It is too abstract for this which is why I say it is only a philosophical idea. Therefore, it can’t be a scientific theory of aging.
Thirdly, I didn’t criticize Sinclair with this statement because he actually has a scientific theory of aging. The main point of his theory is that DNA double-strand breaks cause the hallmarks of aging. DNA breaks are objective because we can empirically observe them. Furthermore, it is possible to empirically falsify his theory. For instance, if we find a way of prevent DNA breaks or make them less likely and some hallmarks of aging stay unchanged, then his theory would be falsified.
Notice that I don’t claim that his theory will really be falsified. However, to count as a scientific theory, clear empirical tests must exist that would allow us to falsify the theory. This doesn’t apply to the damage theory of aging.
Thank you also for your reply, Michael.
Yes, I don’t disagree that for a theory to abide to the scientific method, it usually needs to be quantifiable, measurable and predicting of repeatable phenomena. I have nothing to say against that. I did a BA in Forestry so I kind of know natural sciences, although I followed a different path 20 years ago and became a film director and cinematographer in the film industry, studies of which I did my MA.
As you can see by my first comment, also I don’t have a lesser opinion about philosophy, when compared to science as science itself evolved from philosophy both historically and well… Philosophically. Some schools of philosophical thought like Phenomenology, where started by great mathematicians like Edmund Husserl.
The only thing I was trying to say, and you showed me that you agree with me, is that Sinclair may have made a book for laymen with Lifespan where he may use terms like “damage” but the background for the laying out of that book was his scientific work, which I’m sure is not based in subjective language. He is after all the co-founder of the Aging scientific journal and of The Academy of Health and Lifespan Research.
Best,
Miguel
“First, why don’t cells simply make more repair enzymes? For instances, if Sir2 doesn’t sometimes find its way back to its original position, why not simply make more Sir2? In fact, Sinclair has shown that adding an extra copy of SIR2 increases the lifespan (the number of divisions) in yeast cells (p. 48).”
-It is you who is contradicting yourself here. You just said that he said that adding an extra copy of SIR2 increases the lifespan. As for the other questions:
“But did anyone really do the math here?”
-Maybe not yet, but this does not mean it is wrong.
“What exactly would be the biological advantage if a yeast cell can create more copies of its DNA because it lives longer?”
-Apparently none, this is why they don’t create more copies.
I don’t understand where you see a contradiction in my argument. It goes like this:
My question is if Neo-Darwinism is correct and evolution is all about replication, how can it be too expensive to create just one extra protein if this this keeps the so important replication process going?
You are right, the fact nobody did the math does not make Sinclair’s claim wrong. However, because this theory seems so implausible, I wish someone tested this claim empirically. All you have to do is engineer yeast cells that build more Sir2 and see if those cells replicate more efficiently than normal yeast cells. Provided that both cell colonies receive the same amount food, if the engineered cells replicate faster, claim 3 would be falsified and Sinclair’s theory would be in trouble.
Yes, I agree with you. There should be experiments to test if the energy cost is the same or if it is higher. If it is the same, then the theory is in trouble. If it is a little higher then I believe the theory still stands, considering that primordial earth wasnt a place full of resources (according to him).
But I believe there is more to it. I’m not a biologist or biochemist, so I dont know how to devise an experiment to disprove his ideas. But I guess somebody would have done it by now if it was that simple.
Anyway, I still think his theory deserves a lot of credit because it has produced empirical results in some life forms. He had an idea and this idea generated many experiments whose predictions were confirmed. I see that as a big win so far. As for possible shortcomings, there are certainly a few, there is always. Still I think his research is very promising.
There are only a couple of scientists doing research with sirtuins in yeast and I doubt that they have interest in disproving Sinclair’s claim about the energy costs.
The argument about the conditions in primordial times is not valid because if building more Sir2 would bring and evolutionary advantage, yeast would have adapted since then.
The main reason why I find this very common claim about the additional cost for repairing damage implausible is that there are always times when plenty of food is available and organisms could use this additional energy to repair all the accumulated damage thereby reversing their age. That more available food makes most animals age faster, is one of the many empirical facts that make wear-and-tear theories of aging implausible.
Don’t get me wrong. I am a big fan of Sinclair’s work. I think he is a brilliant experimentalist. Sometimes I just have problems following his more theoretical conclusions.
Yeah, I’m reading the book and I didn’t understand his argument in the beginning. This is why I looked for an explanation and found your website. It seems a little contradictory indeed because it is not the damage itself that causes aging but the lack of sirtuins in the epigenome. So I found it really weird when he said that putting more stress in the body would have the effect of increasing aging. It seemed counterintuitive, but so many things in science are. After I finish the book and review his theory I will be able to make a better judgment of his theory, I guess. What do you think about Aubrey de Grey? Do you know him? His causes of aging are different from the hallmark causes that Sinclair says that the scientific community agrees on. de Grey has a more practical approach, focused on solving the problem of aging with biotech, whereas Sinclair (and I suspect the rest of the field) is more into biochemistry.
I think what he says about stress is consistent. Stress causes damage which causes aging. Moderate stress makes cells hunker down and focus on repairing damage. Thus, moderate stress slows aging, too much stress accelerates aging. Where I can’t follow is his claim that energy costs prevent cells from repairing damage.
Of course I know Aubrey de Grey. I guess everyone who is interested in the biology of aging does. I have even more problems with his theory that damage caused by metabolism (in particular in the mitochondria) is the main cause of aging which is the old view that more and more gerontologists seem to abandon now. I feel that Sinclair is with his focus on the epigenome more on the pulse of cutting edge anti aging research.
I don’t think that Sinclair’s research is less practical. In my next post I will blog about his attempt of using some of the Yamanaka factors to reset the clock of aging which is currently the most promising approach in this field.
Nice! I know Aubrey but I have never read about his theory in detail, only very superficially. I agree that the Yamanaka factors seem to be the most promising ones. They are also the most exciting ones, the idea of reversing aging is just amazing! I’m not old, I’m 27, but even at my age I think the only possibility of having a very long lifespan is if there is some sort of rejuvenation therapy like this one. Otherwise, I believe it’s too late haha I’m looking forward to your post on the Yamanaka factors.
I found this post the same way as Pedro – I am glad I wasn’t the only one who didn’t understand Sinclair’s “Magna superstes” example!
Michael, can I ask where you got the Sir-2 getting “lost” detail from? This causes the explanation to make so much more sense, but I just double checked and it is definitely not mentioned in Sinclair’s explanation or the accompanying diagram.
Conrad, thanks! The thing with the lost Sir2 is in the book. For example, on p. 47:
Sinclair, David . Lifespan: Why We Age—and Why We Don’t Have To (p. 47). Atria Books. Kindle Edition.
This is a good enough idea, but fortunately similar ideas have been tossed around in biology and the evidence for them is much weaker than for aging being a “bug”, although some in the field still think that programmed aging is a better explanation than damage theories. Giving a full rundown on this would exceed this format, but the key word to look up would be “selective shadow”; although it might stretch credulity that such a miniscule cellular process could make an evolutionarily decisive difference in energy expenditure, like you said, we are missing the math here (that is, the numbers on which such math could operate in the first place) – and we will likely never have it.